Part six
NEW SINGULARITY BRANCH (Phosphorus atom as central atom)
PHOSPHATE MOLECULES PO4; HPO4; H2PO4; H3PO4; H4PO4; H5PO5.....
The chemical process can change the content, with the chemical synthesis of the Phosphorus atom.
The Carbon or Phosphorus atoms, as central atoms, determine the kind of molecules.
The beginning of the next singularity is determined by the number of electrons. See Figure 149
Figure 149
| Molecule content | ||||||
| Periphery atoms | Central atoms | |||||
| atom | 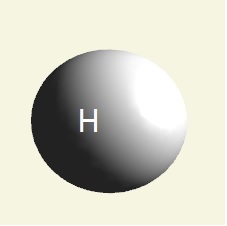 |
 |
 |
 |
 |
|
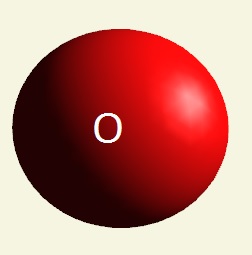
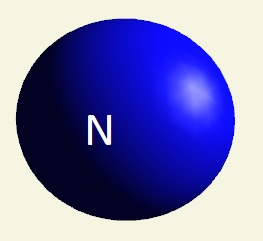
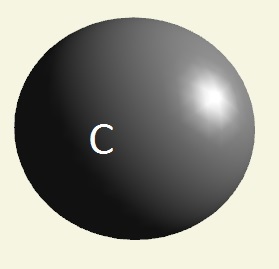
| atom | Hydrogen | Oxygen | Nitrogen | Carbon | Phosphorus | |
| electrons | 1 | 2 - 6 | 2 - 5 | 2 - 4 | 2 - 8 - 5 | |
| mising electrons | 7 | 2 | 3 | 4 | 3 | |
| singularity | 1 | 2 | 3 | 4 | 5 | |
The electron bond is the sum of electron number + missing electrons number = 8.
The singularity number is the distance from the zero point.
ANALYTICAL CHEMISTRY OF THE PHOSPHATE MOLECULE
See Analytical Chemistry
See Phosphorus
See Phosphate molecule.
Two Hydrogen peroxide molecules + Carbon atom → Creatinine molecule (Singularity four - Carbon electrons).
Two Hydrogen peroxide molecules + Phosphorus atom → Phosphate molecule (Singularity five - Phosphorus electrons).
Chemical transformations of the Creatinine molecule, including Phosphorus atom and excluding Carbon and Hydrogen atoms.
(See Figure 150)| Singularity 4 | 2H2O2 + C → CH4O4 | Creatinine molecule CH4O4 | See Figure 150 |
| Singularity 5 | 2H2O2 + P → PH3O4 excludes H | Phosphate molecule H3PO4 | See Phosphoric acid. |
Figure 150
| Molecule content | ||
| Molecule | 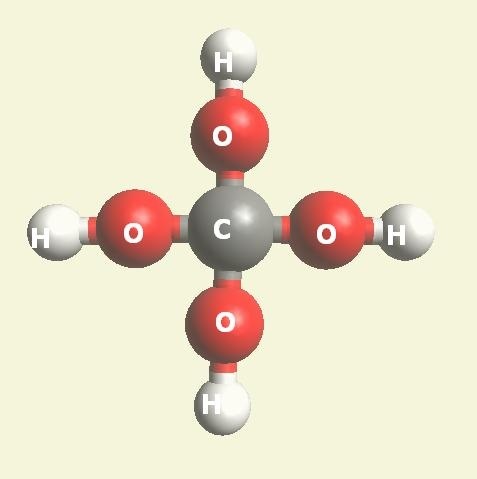 |
 |
| Creatinine molecule | Phosphate molecule | |
| singularity | 4 | 5 |
Hydrogen atoms singularity (prefix example)
| singularity | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| prefix | mono | di | tri | tetra | penta | hexa | hepta | octa | nona | deca |
Chemical transformations of Phosphate molecules, including Hydrogen atoms.
| Phosphate molecule PO4 |
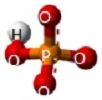
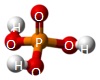
A Phosphate molecule.PO4 (See Figure 151)
| Figure 151 |  |
A Phosphate molecule. PO4 includes H (Hydrogen atom) and becomes a Hydrogen phosphate molecule HPO4.
| PO4 + H → HPO4 |
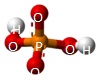
A Phosphate molecule. PO4 includes 2H (Hydrogen atoms) and becomes a Dihydrogen phosphate molecule H2PO4.(See Figure 152)
| PO4 + 2H → H2PO4 |
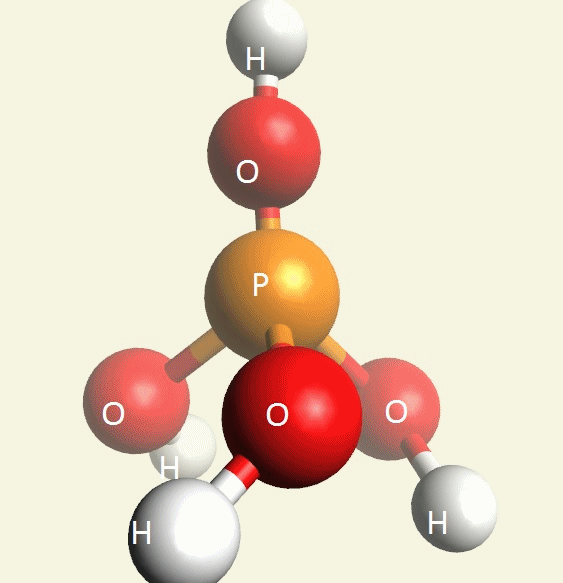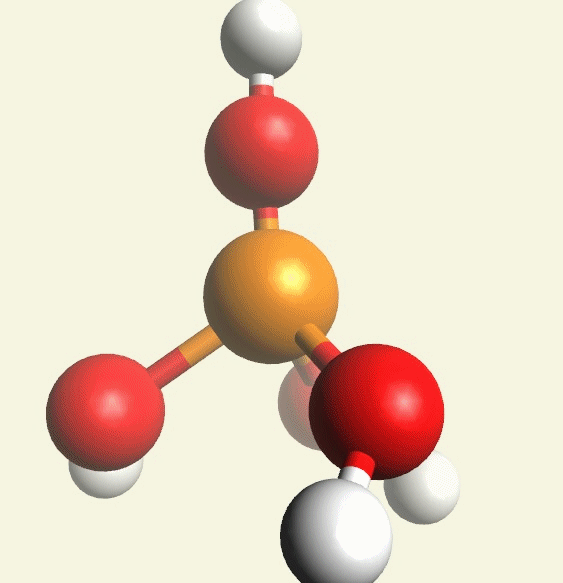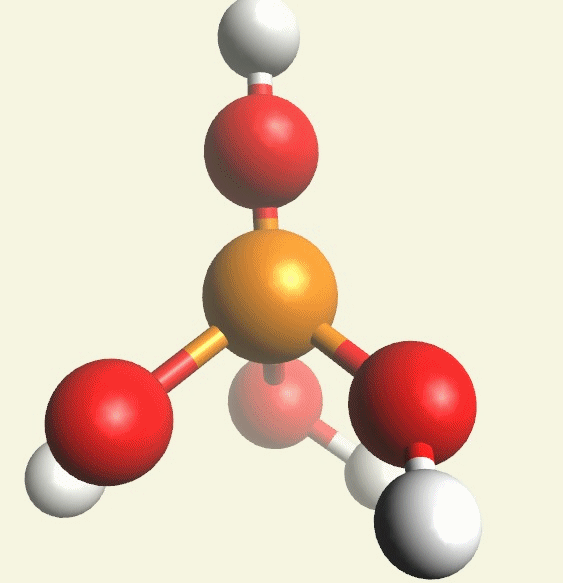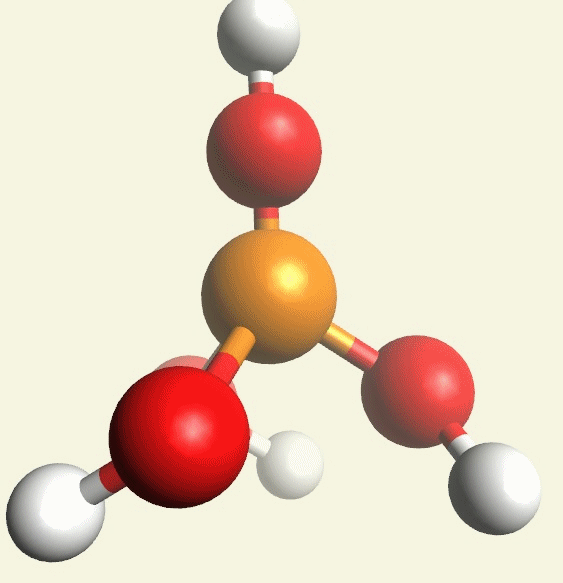
A Phosphate molecule. PO4 includes 3H (Hydrogen atoms) and becomes a Trihydrogen phosphate molecule H3PO4.(See Figure 152)
| PO4 + 3H → H3PO4 |
A Phosphate molecule. PO4 includes 4H (Hydrogen atoms) and becomes a Tetrahydrogen phosphate molecule H3PO4.(See Figure 152)
| PO4 + 4H → H4PO4 |
A Phosphate molecule. PO4 includes 5H (Hydrogen atoms) and O (Oxygen atom) and becomes a Pentahydrogen phosphate molecule H5PO5.(See Figure 152)
| PO4 + 5H + O → H5PO5 |
Figure 152
| Molecule content | ||||||
| Figure 152 |  |
 |
 |
 |
 |
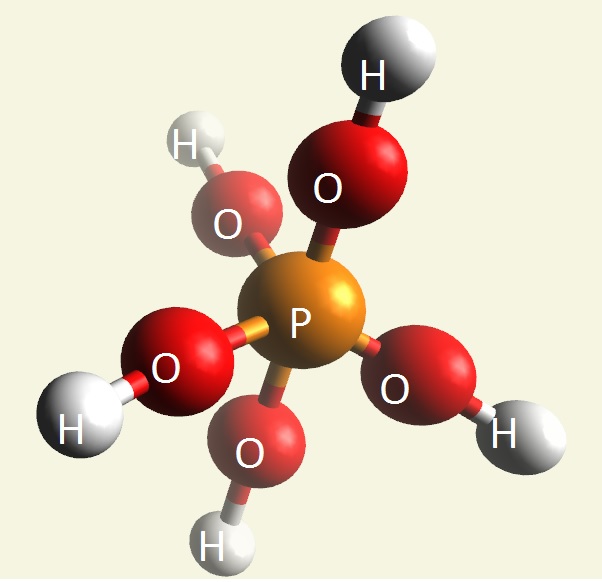 |
| molecule | PO4 | HPO4 | H2PO4 | H3PO4 | H4PO4 | H5PO5 |
| Hydrogen atoms | PO4 | +H | +2H | +3H | +4H | +5H |
| sin | 0 | 1 | 2 | 3 | 4 | 5 |
SINGULARITY LOW - PHOSPHATE MOLECULES CONTENT ANALYZER
Example....
Write velue of blood Phosphate in the field
(example ∑ 1.7 mmol/l.)